A Ketimide-Stabilized Palladium Nanocluster with a Hexagonal Aromatic Pd7 Core | Inorganic Chemistry

SOLVED: Rhodium has a density of 12.41 g>cm3 and crystallizes with the face-centered cubic unit cell. Calculate the radius of a rhodium atom.

Problem.docx - Problem #1: Palladium crystallizes in a face-centered cubic unit cell. Its density is 12.023 g/cm3. Calculate the atomic radius | Course Hero
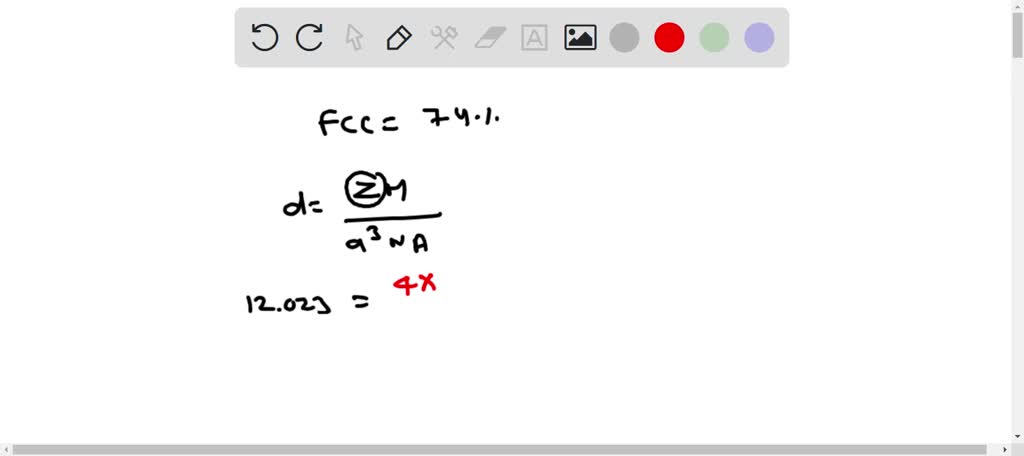
SOLVED: Palladium (at. wt. = 106) crystallizes in a face-centered cubic unit cell. Its density is 12.023 g/cm3 . Calculate the atomic radius of palladium and its packing efficiency.
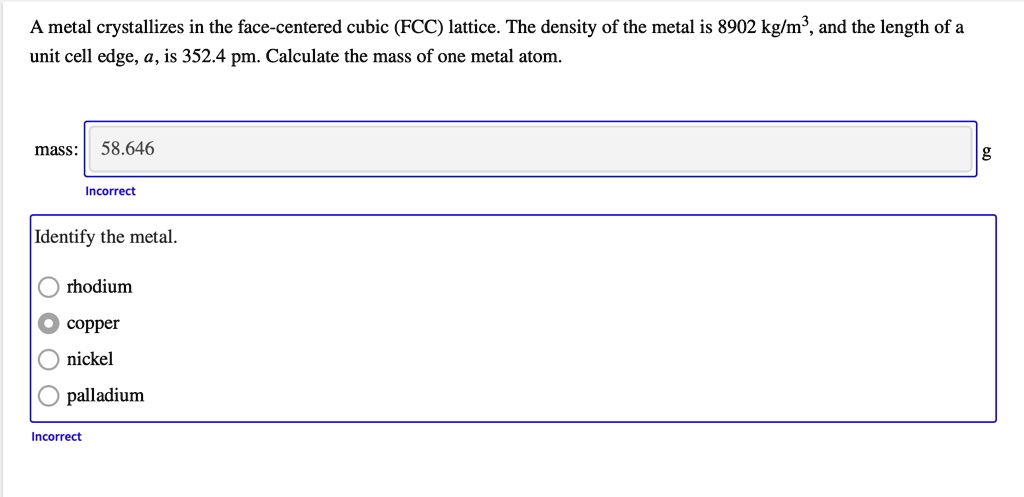
SOLVED: A metal crystallizes in the face-centered cubic (FCC) lattice. The density of the metal is 8902 kglm , and the length of a unit cell edge, a,is 352.4 pm. Calculate the

Interactions between Hydrogen and Palladium Nanoparticles: Resolving Adsorption and Absorption Contributions - Moumaneix - 2023 - ChemElectroChem - Wiley Online Library

roblem.docx - problem #1: Palladium crystallizes in a face-centered cubic unit cell. Its density is 12.023 g/cm3. Calculate the atomic radius of | Course Hero

Document - Problem #1: Palladium crystallizes in a face-centered cubic unit cell. Its density is 12.023 g/cm3. Calculate the atomic radius of palladium. | Course Hero
PLEASE HELP! 80 points!! A metal crystallizes in the face‑centered cubic (FCC) lattice. The density of the - Brainly.com

A metal crystallizes in the face-centered cubic unit cell with an edge length of 320 pm. \\ A. What is the radius of the metal atom? B. The density of the metal